The startup of the former co -founder of Neuralink has received approval of the US Food and Medicines (FDA) for a new type of brain implant that does not penetrate deep into the brain tissue , but is installed on its surface, reports Bloomberg .
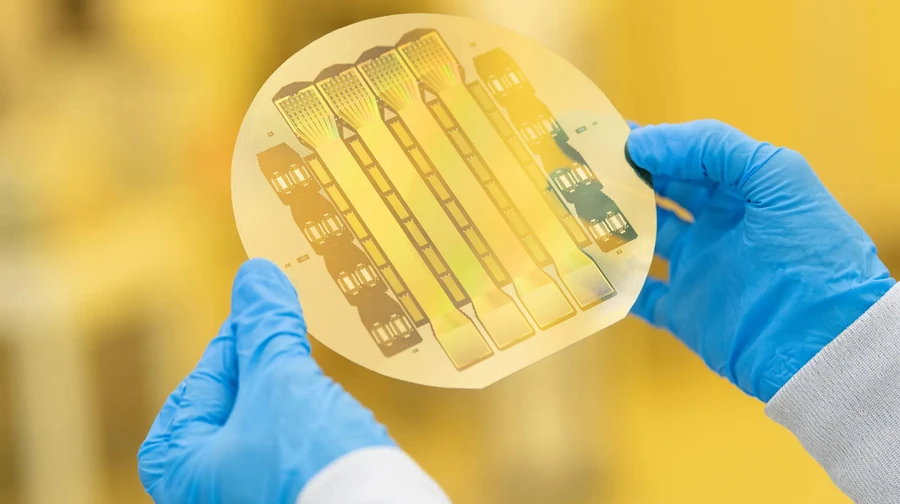
Philadelphia Precision Neuroscience implanted its thin film in a 63 -year -old patient with Parkinson Fisher 's Parkinson's disease early this month. The surgeon placed a lining that is thinner than human hair, which was built in 1024 microelectrodes directly on the surface of the patient's cortex. After a short training, Fisher could control the robotic hand simply by thinking about the left hand movement, the impulses were transmitted through a new implant.
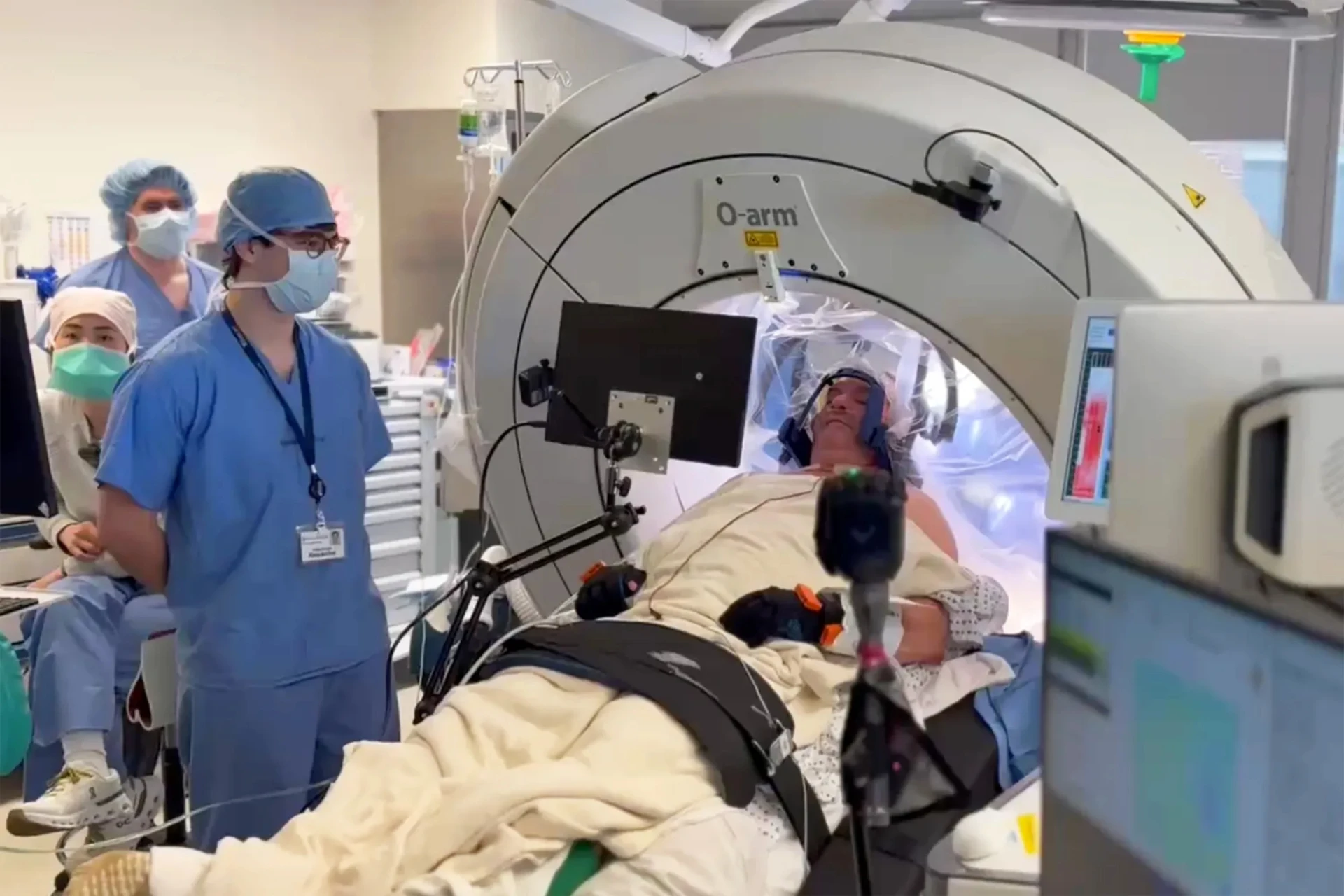
"It was an extraordinary experience," Fisher said, a retailer from West -Chesster, Pennsylvania. Through tremor, it is difficult for him to perform simple daily tasks, such as printing or opening banks, so he hopes that this technology will return to his subtle control of movements one day.
Last month, the FDA granted the implant permit for invasive clinical use for up to 30 days for recording, monitoring and stimulating brain activity . Approval is based on preclin trials. Further clinical studies will help to expand its use. Similar approval earlier received a Neuroport Array device Blackrock Neurotech .
Precision Neuroscience was founded in 2021 by Michael Meiger and neurosurgeon Benjamin Rapoport , who had previously helped to create Neuralink Ilona Mask. Unlike an implant in the form of a chip with "threads" Neuralink, the precision lining can be installed and removed without residual damage to the brain. The device with an area of about one square inch (6.45 square centimeters) has already been tested at least 37 patients in five leading US medical centers, and about a third of establishments conducting complex brain surgery have been interested in this technology.
"We were pleasantly surprised by demand," Rapoport said. The company plans to focus this year on collecting additional patients to improve software that converts electrical signals into detailed brain activity.
These high -precision cards give surgeons and researchers "microscopic" level of detail of neural function, according to Nitin Tandon's neurosurgeon from Uthealth Houston. Tannon, which is not associated with precision or other brain -computer interface companies, believes that this technology can improve operation planning - for example, to avoid zones responsible for movements during epilepsy surgery - and to accelerate the study of speech and cognitive functions.
Precision expects to start the commercial distribution of its implant in 2026. In the future, the company seeks to use its development not only during surgical procedures, but also to create future carbon or implanted systems that will allow people with paralysis to communicate or control devices.
Currently, Fisher enthusiastically recalls his short contact with a robotic hand, joking that he had a "friendship" with an inactive object. "I even made a cam from her," he said. "There was a sense of real connection with the fact that he was not living - but this feeling was very real."

